This is the first article of a series looking at electrical installations in medical locations and will consider the scope of Section 710 of BS 7671, including some of the design requirements.
Consideration will also be given to the use of system Grouping and how the classification of a safety service supply given in Section 560 is applied to such medical locations.
The risk to a patient is greatly increased in a medical location due to:
The scope of Section 710 outlines particular requirements applicable to patient healthcare centres and facilities including:
Healthcare-related construction contracts must also meet the requirements specified in the supporting information for healthcare premises outlined in the Health Technical Memorandum (HTM) 0601 Electrical services supply and distribution, and 06-02 Electrical safety guidance for low voltage systems, published by the Department of Health.
Equivalent guidance documents are available for the devolved administrations: Scotland (SHTM), Wales (WHTM) and Northern Ireland (HTM) (Note 6 to regulation 710.1 refers).
Although, there are often subtle differences in the approach between BS 7671 and the specific HTM, the designer should always comply with the requirements of BS 7671. Where the standard does not provide sufficient information on a particular matter or detail, guidance in the relevant HTM should then be applied. However, such guidance should not contradict the requirements of BS 7671.
The classification of a medical location is typically based on the:
To ensure safety in a medical location, where the location is tobe used for multiple procedures or medical treatments, the requirements applicable to the most onerous usage should be applied.
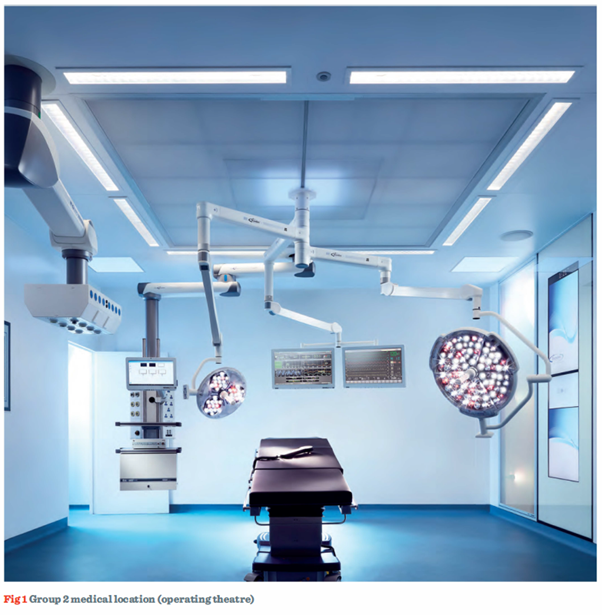
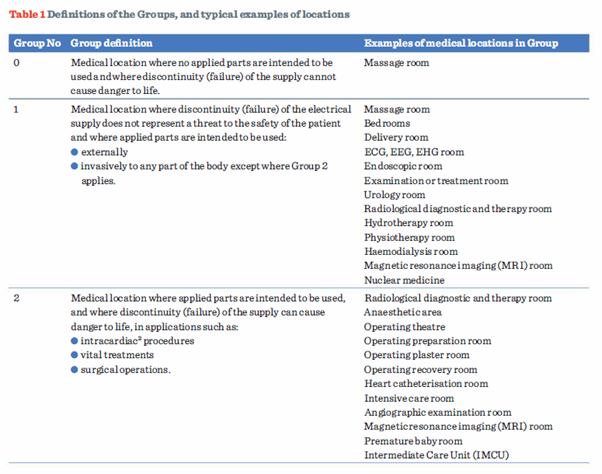
It remains the responsibility of the designer in consultation with the relevant clinical staff to determine the appropriate Group for a particular location (710.3). It should also be borne in mind that the listing is not definitive.
Regulation 560.4.1 provides the classification of supplies for safety services. Section 710 requires such types of automatic supplies for safety services to be operational within a maximum changeover time designated for a particular area.
Table A710 of the annex to Section 710 provides guidance on the maximum permissible changeover times in typical medical locations. For example, within bedrooms and massage rooms a changeover time greater than 0.5 s and not exceeding 15 s is recommended while a time not exceeding 0.5 s is recommended for supplies to luminaires and life-support equipment in locations such as operating theatres.
Care should be taken to ensure that any such installations, or necessary work, do not influence or compromise the level of safety imposed by Section 710, including the effects from:
Subsequent technical articles will consider the functionality of medical IT systems, and the requirements for achieving shock protection in such locations. We would like to acknowledge and thank Brandon Medical for providing the image used within this article.
Footnotes
1. Applied parts are parts of medical electrical equipment that necessarily come into physical contact with the patient in normal use, for medical electrical equipment or a medical electrical system to perform its function. See Definitions relating to medical locations.
2. An intracardiac procedure is where an electrical conductor is placed within the heart of a patient or is likely to come into contact with the heart, such conductor being accessible outside the patient's body. In this context, an electrical conductor includes insulated wires such as cardiac pacing electrodes or intracardiac ECG electrodes, or insulated tubes filled with conducting fluids. See Definitions relating to medical locations.
Introduction
The intention of Section 710 is to provide enhanced reliability for the electrical installation for, and greater electrical safety within, the medical environment for both patients and medical staff.The risk to a patient is greatly increased in a medical location due to:
- the threat from failure of the supply to medical equipment, especially that used for life support; and
- the natural reduction in body resistance inherent with open wounds, intrusive procedures and subsequent decline in patient defensive capacity either from induced medication or while anaesthetised.
The scope of Section 710 outlines particular requirements applicable to patient healthcare centres and facilities including:
- hospitals;
- private clinics;
- medical and dental practices;
- dedicated medical rooms in the workplace; and
- patient medical research establishments.
Healthcare-related construction contracts must also meet the requirements specified in the supporting information for healthcare premises outlined in the Health Technical Memorandum (HTM) 0601 Electrical services supply and distribution, and 06-02 Electrical safety guidance for low voltage systems, published by the Department of Health.
Equivalent guidance documents are available for the devolved administrations: Scotland (SHTM), Wales (WHTM) and Northern Ireland (HTM) (Note 6 to regulation 710.1 refers).
Although, there are often subtle differences in the approach between BS 7671 and the specific HTM, the designer should always comply with the requirements of BS 7671. Where the standard does not provide sufficient information on a particular matter or detail, guidance in the relevant HTM should then be applied. However, such guidance should not contradict the requirements of BS 7671.
Assessment of general characteristics
Part 3 of BS 7671 outlines the characteristics that need to be assessed for all electrical installations. The designer having responsibility of the electrical installation, within a medical location, is required to determine both the appropriate Group into which a particular medical location falls, and the classification that is to be applied to the electrical safety services in those locations.The classification of a medical location is typically based on the:
- type of physical contact between the patient and the applied parts' of medical equipment (ME) or ME systems;
- threat to patient safety in the event of a loss of supply; and
- purpose for which the location is to be used.
To ensure safety in a medical location, where the location is tobe used for multiple procedures or medical treatments, the requirements applicable to the most onerous usage should be applied.

Grouping
The guidance given in Table 1, highlights the definitions for Groups 0, 1 and 2 locations identified in Part 2 of BS 7671, and provides, based on Annex A710 of Section 710, some examples of rooms likely to be included in each Group.
It remains the responsibility of the designer in consultation with the relevant clinical staff to determine the appropriate Group for a particular location (710.3). It should also be borne in mind that the listing is not definitive.
Classification of a safety service
As described previously, the failure of an electrical supply in a medical location could cause danger to life. Appropriate safety services are therefore necessary to prevent such risk, and may typically include the provision for a standby supply for an operating theatre light.Regulation 560.4.1 provides the classification of supplies for safety services. Section 710 requires such types of automatic supplies for safety services to be operational within a maximum changeover time designated for a particular area.
Table A710 of the annex to Section 710 provides guidance on the maximum permissible changeover times in typical medical locations. For example, within bedrooms and massage rooms a changeover time greater than 0.5 s and not exceeding 15 s is recommended while a time not exceeding 0.5 s is recommended for supplies to luminaires and life-support equipment in locations such as operating theatres.
Further design considerations
The requirements for other sections in Part 7 of BS 7671 may also need to be considered, particularly where it is necessary to modify the electrical installation due to a change of utilisation for the location. For example, where such intended work includes areas containing a bath or shower (701), or for mobile medical or transportable units (717).Care should be taken to ensure that any such installations, or necessary work, do not influence or compromise the level of safety imposed by Section 710, including the effects from:
- fault current;
- electromagnetic interference (EMI);
- electromagnetic compatibility (EMC); or
- fire.
Summary
This article outlines some of the considerations given in the scope of Section 710 of BS 7671 for various types of medical facilities. The requirements for specific Groups and safety service supply classifications utilised within a medical/healthcare environment have also been considered.Subsequent technical articles will consider the functionality of medical IT systems, and the requirements for achieving shock protection in such locations. We would like to acknowledge and thank Brandon Medical for providing the image used within this article.
Footnotes
1. Applied parts are parts of medical electrical equipment that necessarily come into physical contact with the patient in normal use, for medical electrical equipment or a medical electrical system to perform its function. See Definitions relating to medical locations.
2. An intracardiac procedure is where an electrical conductor is placed within the heart of a patient or is likely to come into contact with the heart, such conductor being accessible outside the patient's body. In this context, an electrical conductor includes insulated wires such as cardiac pacing electrodes or intracardiac ECG electrodes, or insulated tubes filled with conducting fluids. See Definitions relating to medical locations.



