This article introduces the types of batteries currently available, including the technical terms used, and their typical applications.
The construction and chemical processes during the charging/discharging cycle of batteries is outside the scope of this article. This article will be complemented using a number of multiple-choice questions.
Batteries are the primary power source for many electronic and wireless devices, such as mobile phones, laptops, cordless power tools, etc. They are also employed, on a somewhat larger scale, within the electrical installations to provide as a back-up source for many safety services (see Regulation 560.1 of BS 7671) and, more recently, in electrical energy storage systems (EESS).
(i) Primary cells – which are non-rechargeable
(ii) Secondary batteries – which can be recharged.
A primary cell is a battery that is designed to be used only once and then discarded.
The functionality of a secondary battery1 is exactly the same as that of a primary cell, but it is typically a group of one or more cells arranged in a series/parallel network so that the voltage or current respectively (or both) can be raised to desired levels. It is also capable of being recharged.
How batteries are interconnected will be covered in a subsequent article.
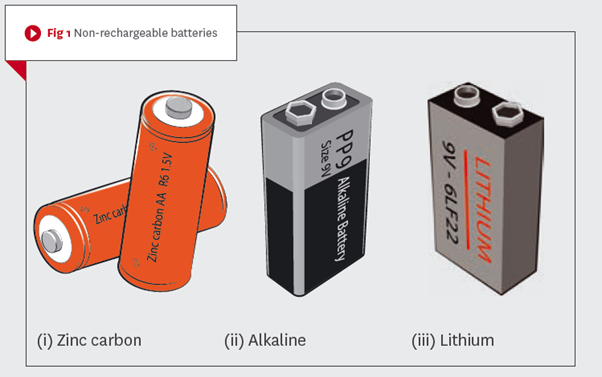
The alkaline battery, when compared to the zinc-carbon, has a higher energy density3 and a longer shelf life. However, the lithium battery out-performs both alkaline and zinc-carbon batteries by a considerable margin. Its energy density is five times greater than the alkaline battery, and 10 times greater than the zinc-carbon battery.
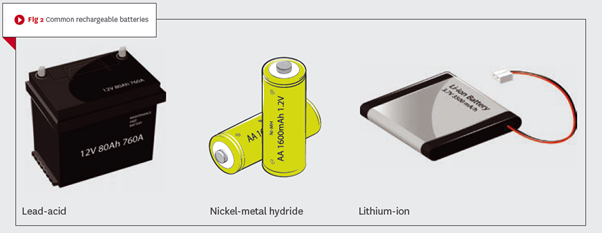
Examples of the common types of rechargeable batteries (Fig 2) include: lead-acid, nickel-cadmium (Ni-Cd), nickel-metal hydride (NiMH), lithium-ion (Li-ion), and rechargeable alkaline.
Lead-acid batteries
The lead-acid is the oldest battery technology still in common use. They are used mostly for larger power applications where weight is of little concern. They are typically used in emergency lighting and for the back-up supply for fire alarm and detection systems, and in some UPS4 systems.
They can be used in home energy storage systems (EESS) as a cost-effective option, but when compared with other types, such as the Li-ion, they have a lower lifespan and depth of discharge (their low energy density means they cannot be stored in a discharged condition).
Nickel-cadmium
Although these batteries are very rarely used today, they are relatively cheap and their discharge rate is very low when compared to Ni-MH batteries. One disadvantage with the Ni-Cd is they have a ‘memory’. This means that the battery will ‘remember’ the point in its charge cycle where recharging began. Therefore, during subsequent uses, voltage will drop at that point as if it had been discharged. It is more viable to use a Ni-Cd battery continuously until it is totally depleted before recharging. When used in conjunction with manufacturer’s instructions, a nickel-cadmium battery can last for 1,000+ cycles before losing capacity.
Nickel-metal hydride
These batteries are preferable to Ni-Cd batteries because of their lower environmental impact. This includes the materials used to construct the batteries and the safe disposal of spent batteries. They have a greater terminal voltage than for Ni-Cd batteries, but less than for alkaline batteries.
The modern Ni-MH battery offers up to 40 percent higher energy density compared to Ni-Cd, powering devices for longer for an equivalent rated battery. However, it is less durable than the Ni-Cd and cycling under heavy load and storage at high temperature reduces its service life. The Ni-MH suffers from a considerably greater self-discharge when left unused than a Ni-Cd.
One of its main limitations is that it generates more heat while charging and requires a longer charge time than the Ni-Cd. The trickle charge is critical and must be carefully controlled
.
Some advanced chargers provide a ‘step-differential charge’ which allows for an initial fast charging state before reaching a threshold voltage; at which point the battery is required to cool before entering a state of trickle charge, where the charging current is continually reducing until the battery is fully charged.
Due to their ability for high energy release, these batteries incorporate a fuse that operates on high current and temperature, which may render the battery useless5.
Typically, where individual cells are arranged to form a battery – such as those used in electric vehicles – precautions need to be taken to reduce thermal runaway between cells and prevent the risk of fire. Such precautions generally include thermal protection padding to limit the transfer of heat between cells, and fire blocking foam to suppress any fire.
While the Lithium-ion battery may provide more voltage, it does so at significantly higher financial cost compared to a Ni-Cad battery. However, both will perform for roughly the same number of charge/discharge cycles.
*****
1 Often called storage batteries.
2 Terminal voltage is the open-circuit voltage which is available before a load is connected.
3 Energy density is the amount energy stored per unit volume.
4 UPS – An uninterruptible power source is an item of electrical equipment that provides emergency power to a load upon mains power failure.
5 Some types may incorporate a reset switch.
*****
Battery capacity – this is a measure of the charge stored by the battery and is determined by the mass of active material contained in the battery. The battery capacity represents the maximum amount of energy that can be extracted from the battery under certain specified conditions – namely current loading, temperature and battery age, amongst other things.
As energy is the product of power and time (P × t), it is expected that the energy stored in a battery would be measured in Watt-hours (Wh). However, since Wh = U × I × h and because the voltage is usually fairly constant, the unit representing battery capacity becomes Ah or mAh. The Ah rating on its own means very little without knowing what hour rate was used.
C-Rate – this is the amount of current that a battery can deliver (or discharge) over a given time and is termed its C-rating. It is closely related to the battery Ah capacity.
Example: A battery is marked as 22 000 mAh (22 A) at 22.5 V and 20 C.
Maximum discharge current = mAh × C – rate = 22 × 20 = 440A
The battery would be incapable of supplying this amount of current for any appreciable length of time as the battery voltage would very quickly diminish. A more acceptable discharge rate would be 1 C (22 A) or less.
Table 1 shows how different C ratings affect the capacity of a 1 Ah rated battery.
Note: The higher the C-rating, the greater the current that can be drawn from the battery.

Nominal voltage – batteries are typically marked with a nominal voltage, as shown in Fig 1 and Fig 2. However, a fully charged battery may have a higher open-circuit voltage (OCV) when tested at the terminals.
Charging current – this is the maximum current that can be applied to charge a battery. Some high performance batteries may allow charging to be carried out at a higher C rating with minimal stress, although manufacturer’s instructions must be taken into consideration.
Charging voltage – this is the maximum voltage that should be applied to the battery to efficiently charge it. As the battery’s voltage starts to rise, the charging current decreases. The charging voltage available must be greater than the battery’s voltage rating.
Discharging current – this is the current that can be drawn from the battery and delivered to a load. If the current drawn by the load is greater than the rated discharging current, the battery drains very quickly, causing the battery to heat up rapidly. For some batteries, this could lead to them exploding. It is essential that manufacturer’s instructions are followed closely when determining the amount of current drawn by the load, as well as the maximum discharging current a battery can withstand.
Shelf life – this defines the time period a battery can stay fully charged before use and then be used for a rated time period. Shelf life is mainly considered for non-rechargeable batteries because those are generally required for immediate use. For rechargeable batteries, even if the shelf time is less, they can be recharged.
Cut-off voltage – the voltage at which the battery can be considered as fully discharged. Further discharging may cause permanent damage to the battery, in some cases leading to a fire or explosion.
Cycle life – if a battery is fully charged and then is discharged to 80% of its actual capacity, the battery is said to have completed one cycle. The number of such cycles that a battery can charge and discharge defines the cycle life. The more the cycle life the better will be the battery’s quality. However, if a battery is discharged to, say, 40% of its actual capacity (considering the battery is fully charged initially), it cannot be considered as a cycle life.
Depth of Discharge (DoD) – the DoD determines the overall cycle count of the battery. A battery having a small discharge or low DoD will typically last much longer, due to enduring less stress.
It may be considered as a fraction or percentage of the capacity which has been removed from the fully charged battery. DoD is defined as the capacity that is discharged from a fully charged battery, divided by battery nominal capacity.
a) The electrochemical reaction is not reversible
b) It can only be charged and discharged once
c) It can only be used as a back-up energy source
d) It can be charged and discharged many times
2. The capacity of a battery is expressed in terms of its:
a) Current rating
b) Voltage rating
c) Ampere hour rating
d) Depth of discharge
3. Which of the following are generally used in a domestic electrical energy storage systems?
a) Nickel-cadmium battery
b) Lead-acid battery
c) Nickel-metal hydride
d) Rechargeable alkaline battery
4. Trickle charging of a storage battery helps to:
a) Increase its loading capability
b) Reduce its reverse capacity
c) Improve its cycle life
d) Keep it in a ready-to-use condition
5. The output voltage of a battery charger is:
a) Higher than the battery voltage
b) Less than the battery voltage
c) The same as the battery voltage
d) Ripple-free low voltage DC
6. A battery casing is marked 25C to 40C. This means that:
a) The battery will discharge in 1 hour when suppling a load current between 25 A and 40 A
b) The battery will discharge in 1 hour at 25 A if the ambient temperature is less than 40 °C
c) 25C is the normal discharge rate and 40C is the maximum burst discharge rate
d) 40 A is the absolute maximum the battery can supply to a load without damage
A primary battery can only be used the once and must not be recharged.
2. Correct option is (c)
The ampere hour (Ah) provides a measurement of battery capacity, although the relationship between battery capacity and the rate of discharge is not a linear one. For example, a 100 Ah battery will not provide 100 A for one hour. It would however provide, say, 20 A for five hours whilst maintaining a voltage close to its terminal voltage.
3. Correct option is (b)
4. Correct option is (d)
5. Correct option is (a)
The rate of current flow when charging a battery in a discharged state can be high.
It is for this reason that ‘smart chargers’ are recommended, which limit the charging current to a safe level throughout the charging period. The charging voltage must be high enough to overcome the battery’s internal resistance to permit the charging current to flow.
6. Correct option is (c)
The charge and discharge rates of a battery are governed by C-rates. If, for example, the capacity of a battery is rated at 5 200 mAh, the maximum discharge current is 130 A (5.2 x 25) but can give a current burst of 208 A (5.2 x 40).
Introduction
Once considered heavy and cumbersome and only capable of delivering a relatively small current in proportion to their size and weight, advances in battery technology have seen the performance of batteries improve significantly.Batteries are the primary power source for many electronic and wireless devices, such as mobile phones, laptops, cordless power tools, etc. They are also employed, on a somewhat larger scale, within the electrical installations to provide as a back-up source for many safety services (see Regulation 560.1 of BS 7671) and, more recently, in electrical energy storage systems (EESS).
Battery types
A battery is an electrochemical energy source that can deliver only DC voltage and current. Batteries are classified into two categories:(i) Primary cells – which are non-rechargeable
(ii) Secondary batteries – which can be recharged.
A primary cell is a battery that is designed to be used only once and then discarded.
The functionality of a secondary battery1 is exactly the same as that of a primary cell, but it is typically a group of one or more cells arranged in a series/parallel network so that the voltage or current respectively (or both) can be raised to desired levels. It is also capable of being recharged.
How batteries are interconnected will be covered in a subsequent article.
Non-rechargeable batteries (primary)
The zinc-carbon battery (Fig 1(i)) is one of the oldest types still in current use. Its terminal voltage2 is slightly higher than is available, size for size, for the alkaline (Fig 1(ii)) or lithium (Fig 1(iii)) battery. Its current output is the lowest of all the popular non-rechargeable battery types, which makes it suitable for small load applications, such as in torches.
The alkaline battery, when compared to the zinc-carbon, has a higher energy density3 and a longer shelf life. However, the lithium battery out-performs both alkaline and zinc-carbon batteries by a considerable margin. Its energy density is five times greater than the alkaline battery, and 10 times greater than the zinc-carbon battery.
Rechargeable batteries (secondary)
This category of battery can be recharged and hence re-used. Though the initial cost is typically more than for a comparable primary battery, when properly used and maintained they do have a significant life span in terms of the number of times they can be discharged/recharged.Examples of the common types of rechargeable batteries (Fig 2) include: lead-acid, nickel-cadmium (Ni-Cd), nickel-metal hydride (NiMH), lithium-ion (Li-ion), and rechargeable alkaline.

Lead-acid batteries
The lead-acid is the oldest battery technology still in common use. They are used mostly for larger power applications where weight is of little concern. They are typically used in emergency lighting and for the back-up supply for fire alarm and detection systems, and in some UPS4 systems.
They can be used in home energy storage systems (EESS) as a cost-effective option, but when compared with other types, such as the Li-ion, they have a lower lifespan and depth of discharge (their low energy density means they cannot be stored in a discharged condition).
Nickel-cadmium
Although these batteries are very rarely used today, they are relatively cheap and their discharge rate is very low when compared to Ni-MH batteries. One disadvantage with the Ni-Cd is they have a ‘memory’. This means that the battery will ‘remember’ the point in its charge cycle where recharging began. Therefore, during subsequent uses, voltage will drop at that point as if it had been discharged. It is more viable to use a Ni-Cd battery continuously until it is totally depleted before recharging. When used in conjunction with manufacturer’s instructions, a nickel-cadmium battery can last for 1,000+ cycles before losing capacity.
Nickel-metal hydride
These batteries are preferable to Ni-Cd batteries because of their lower environmental impact. This includes the materials used to construct the batteries and the safe disposal of spent batteries. They have a greater terminal voltage than for Ni-Cd batteries, but less than for alkaline batteries.
The modern Ni-MH battery offers up to 40 percent higher energy density compared to Ni-Cd, powering devices for longer for an equivalent rated battery. However, it is less durable than the Ni-Cd and cycling under heavy load and storage at high temperature reduces its service life. The Ni-MH suffers from a considerably greater self-discharge when left unused than a Ni-Cd.
One of its main limitations is that it generates more heat while charging and requires a longer charge time than the Ni-Cd. The trickle charge is critical and must be carefully controlled
.
Some advanced chargers provide a ‘step-differential charge’ which allows for an initial fast charging state before reaching a threshold voltage; at which point the battery is required to cool before entering a state of trickle charge, where the charging current is continually reducing until the battery is fully charged.
Lithium-ion batteries
There are many types of Lithium-ion batteries, and they are used in a wide range of applications. They are generally low maintenance, and can resist the ‘memory effect’ whilst tolerating a wider range of temperatures. Their only serious drawback is their fragility, and the need for a protection circuit to keep them working safely, as required under the IEC 62133-2: 2017 standard when used in portable equipment.Due to their ability for high energy release, these batteries incorporate a fuse that operates on high current and temperature, which may render the battery useless5.
Typically, where individual cells are arranged to form a battery – such as those used in electric vehicles – precautions need to be taken to reduce thermal runaway between cells and prevent the risk of fire. Such precautions generally include thermal protection padding to limit the transfer of heat between cells, and fire blocking foam to suppress any fire.
While the Lithium-ion battery may provide more voltage, it does so at significantly higher financial cost compared to a Ni-Cad battery. However, both will perform for roughly the same number of charge/discharge cycles.
*****
1 Often called storage batteries.
2 Terminal voltage is the open-circuit voltage which is available before a load is connected.
3 Energy density is the amount energy stored per unit volume.
4 UPS – An uninterruptible power source is an item of electrical equipment that provides emergency power to a load upon mains power failure.
5 Some types may incorporate a reset switch.
*****
Technical terms used while dealing with batteries
There are a number of terms used to explain the characteristics of a battery. These terms include:Battery capacity – this is a measure of the charge stored by the battery and is determined by the mass of active material contained in the battery. The battery capacity represents the maximum amount of energy that can be extracted from the battery under certain specified conditions – namely current loading, temperature and battery age, amongst other things.
As energy is the product of power and time (P × t), it is expected that the energy stored in a battery would be measured in Watt-hours (Wh). However, since Wh = U × I × h and because the voltage is usually fairly constant, the unit representing battery capacity becomes Ah or mAh. The Ah rating on its own means very little without knowing what hour rate was used.
C-Rate – this is the amount of current that a battery can deliver (or discharge) over a given time and is termed its C-rating. It is closely related to the battery Ah capacity.
Example: A battery is marked as 22 000 mAh (22 A) at 22.5 V and 20 C.
Maximum discharge current = mAh × C – rate = 22 × 20 = 440A
The battery would be incapable of supplying this amount of current for any appreciable length of time as the battery voltage would very quickly diminish. A more acceptable discharge rate would be 1 C (22 A) or less.
Table 1 shows how different C ratings affect the capacity of a 1 Ah rated battery.
Note: The higher the C-rating, the greater the current that can be drawn from the battery.

Nominal voltage – batteries are typically marked with a nominal voltage, as shown in Fig 1 and Fig 2. However, a fully charged battery may have a higher open-circuit voltage (OCV) when tested at the terminals.
Charging current – this is the maximum current that can be applied to charge a battery. Some high performance batteries may allow charging to be carried out at a higher C rating with minimal stress, although manufacturer’s instructions must be taken into consideration.
Charging voltage – this is the maximum voltage that should be applied to the battery to efficiently charge it. As the battery’s voltage starts to rise, the charging current decreases. The charging voltage available must be greater than the battery’s voltage rating.
Discharging current – this is the current that can be drawn from the battery and delivered to a load. If the current drawn by the load is greater than the rated discharging current, the battery drains very quickly, causing the battery to heat up rapidly. For some batteries, this could lead to them exploding. It is essential that manufacturer’s instructions are followed closely when determining the amount of current drawn by the load, as well as the maximum discharging current a battery can withstand.
Shelf life – this defines the time period a battery can stay fully charged before use and then be used for a rated time period. Shelf life is mainly considered for non-rechargeable batteries because those are generally required for immediate use. For rechargeable batteries, even if the shelf time is less, they can be recharged.
Cut-off voltage – the voltage at which the battery can be considered as fully discharged. Further discharging may cause permanent damage to the battery, in some cases leading to a fire or explosion.
Cycle life – if a battery is fully charged and then is discharged to 80% of its actual capacity, the battery is said to have completed one cycle. The number of such cycles that a battery can charge and discharge defines the cycle life. The more the cycle life the better will be the battery’s quality. However, if a battery is discharged to, say, 40% of its actual capacity (considering the battery is fully charged initially), it cannot be considered as a cycle life.
Depth of Discharge (DoD) – the DoD determines the overall cycle count of the battery. A battery having a small discharge or low DoD will typically last much longer, due to enduring less stress.
It may be considered as a fraction or percentage of the capacity which has been removed from the fully charged battery. DoD is defined as the capacity that is discharged from a fully charged battery, divided by battery nominal capacity.
Multiple-choice questions
1. A primary battery is a battery where:a) The electrochemical reaction is not reversible
b) It can only be charged and discharged once
c) It can only be used as a back-up energy source
d) It can be charged and discharged many times
2. The capacity of a battery is expressed in terms of its:
a) Current rating
b) Voltage rating
c) Ampere hour rating
d) Depth of discharge
3. Which of the following are generally used in a domestic electrical energy storage systems?
a) Nickel-cadmium battery
b) Lead-acid battery
c) Nickel-metal hydride
d) Rechargeable alkaline battery
4. Trickle charging of a storage battery helps to:
a) Increase its loading capability
b) Reduce its reverse capacity
c) Improve its cycle life
d) Keep it in a ready-to-use condition
5. The output voltage of a battery charger is:
a) Higher than the battery voltage
b) Less than the battery voltage
c) The same as the battery voltage
d) Ripple-free low voltage DC
6. A battery casing is marked 25C to 40C. This means that:
a) The battery will discharge in 1 hour when suppling a load current between 25 A and 40 A
b) The battery will discharge in 1 hour at 25 A if the ambient temperature is less than 40 °C
c) 25C is the normal discharge rate and 40C is the maximum burst discharge rate
d) 40 A is the absolute maximum the battery can supply to a load without damage
Answers
1. Correct option is (a)A primary battery can only be used the once and must not be recharged.
2. Correct option is (c)
The ampere hour (Ah) provides a measurement of battery capacity, although the relationship between battery capacity and the rate of discharge is not a linear one. For example, a 100 Ah battery will not provide 100 A for one hour. It would however provide, say, 20 A for five hours whilst maintaining a voltage close to its terminal voltage.
3. Correct option is (b)
4. Correct option is (d)
5. Correct option is (a)
The rate of current flow when charging a battery in a discharged state can be high.
It is for this reason that ‘smart chargers’ are recommended, which limit the charging current to a safe level throughout the charging period. The charging voltage must be high enough to overcome the battery’s internal resistance to permit the charging current to flow.
6. Correct option is (c)
The charge and discharge rates of a battery are governed by C-rates. If, for example, the capacity of a battery is rated at 5 200 mAh, the maximum discharge current is 130 A (5.2 x 25) but can give a current burst of 208 A (5.2 x 40).



